Acetone Polar or Nonpolar Solvent
Acetone has a dipole moment of 288 D and hence is a polar molecule despite possessing characteristics of both the polar and nonpolar substances. Read customer reviews find best sellers.

Is C3h6o Acetone Polar Or Non Polar Youtube
When you place a non-polar molecule in a polar solvent such as oil in water the molecules try to minimize contact with the surface.

. Solvents composed of polar molecules such as water dissolve other polar molecules such as table salt while nonpolar solvents such as gasoline dissolve nonpolar substances such as wax. For example acetone C 3 H 6 O is an organic solvent but is polar as well. It can use its H atom to participate in H-bonding.
Is ethanol polar or nonpolar and why. Free Shipping Site to Store. Nonpolar solvents include alkanes pentane.
Not all organic solvents are nonpolar. Lipids are non-polar organic compounds. Any small molecule cannot be polar or non polar at the same time.
Polar solvents have a positive and a negative charge at different places in their structures and will dissolve other polar substances. Water is a polar solvent. It is classified as the simplest ketone.
See answer 1 Best Answer. A protic solvent has an H atom bound to O or N. Acetone is a polar aprotic solvent.
The values for acetone are µ 288 D and ε 21. Here acetone has been added to water left tube and carbon tetrachloride right tube. Is acetone a nonpolar solvent.
So Is Acetone Polar or Nonpolar. Other polar solvents include acetone acetonitrile dimethylformamide DMF dimelthylsulfoxide DMSO isopropanol and methanol. It has a characteristic odor and flammable in nature.
Register for Free Shipping. Up to 24 cash back solvents and non-polar molecules are soluble in non-polar mostly organic solvents. An Endless Assortment on One Easy-to-Use Site.
Polarity of Solvents Author. Acetone is a relatively polar organic solvent. The bonds between the atoms have very different but measurable electronegativities.
Hence they are soluble in organic solvents such as ethanol alcohol but insoluble in water. The concentration units we have just discussed are used to describe the degree to which a soluble is soluble in a solvent. Many students may have a question regarding whether acetone is polar or not.
Water is a popular example of a polar solvent. So acetone is more polar than say hexane benzene and chloroform but less polar than acetonitrile acetic acid water and a saturated ammonium chloride solution. Ad Browse discover thousands of brands.
Acetone Dicholoroethane Tetrahydrofuran Dicholoromethane Chloroform Diethylether Benzene Toluene Xylene Carbontetrachloride Cyclohexane Petroleum ether Hexane Pentane Non-polar Polar. Ad Business Customers Get Fast Shipping on Thousands of Top Brands and Millions of Products. The dielectric constant is the measure of the polarity of a solvent.
Acetone is another molecular material with both polar and nonpolar characteristics. Is acetone or ethanol more polar. A solvent is a liquid that dissolves a solute.
So acetone is a polar solvent. Liquid acetic acid is a hydrophilic polar protic solvent similar to ethanol and water. There are more and less polar solvents.
The hydrophobic interaction of the carbon in the short chain with water is not great and is overcome by the hydrogen bonding. It exists as a colorless volatile liquid. What is solvent polarity.
With a moderate relative static permittivity dielectric constant of 62 it dissolves not only polar compounds such as inorganic salts and sugars but also non-polar compounds such as oils as well as polar solutes. A polar solvent can dissolve ions and other polar compounds. Up to 24 cash back Is acetone polar or nonpolar solvent Acetone is an organic compound with its chemical formula CH32CO.
The degree that a solvent dissolves a given solute is known as its solubility. Acetone polar or nonpolar. Chloroform is nonpolar because it has a low dielectric constant.
As you can see it has mixed with both the polar water molecules and with the nonpolar carbontetrachloride molecules. A solvent is polar if it has a dipole moment greater than 16 D and a dielectric constant greater than 5. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom.
As a result the dipole moment of Acetone is around 269 D. Acetone exists in a liquid state at room temperature. Why is chloroform a non-polar solvent.
Ethanol extracts the lipid from the crushed solid sample. Ad Save On Acetone Solvent. Polar solvent is a type of solvent that has large partial charges or dipole moments.

Why Is Acetone A Good Solvent Properties Explanation Video Lesson Transcript Study Com
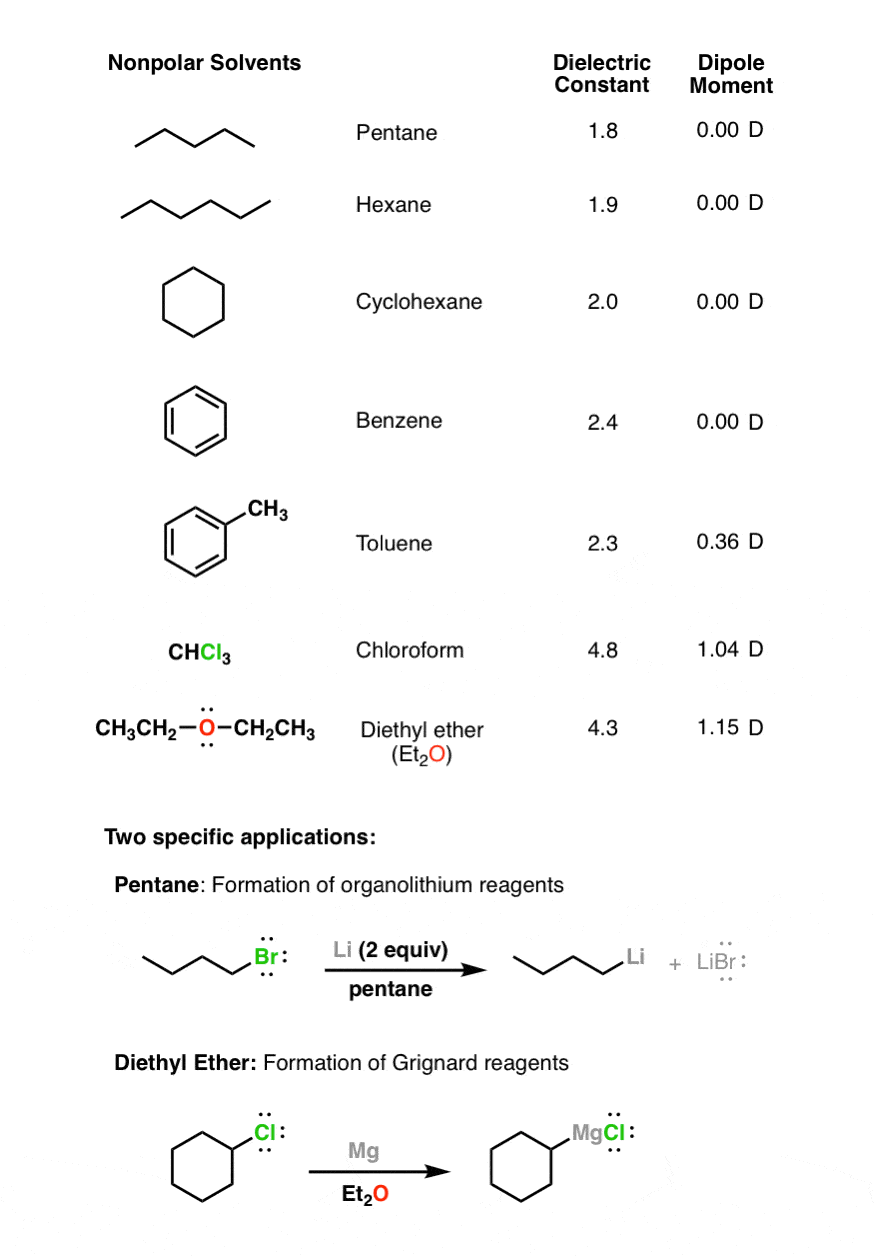
Polar Protic Polar Aprotic Nonpolar All About Solvents
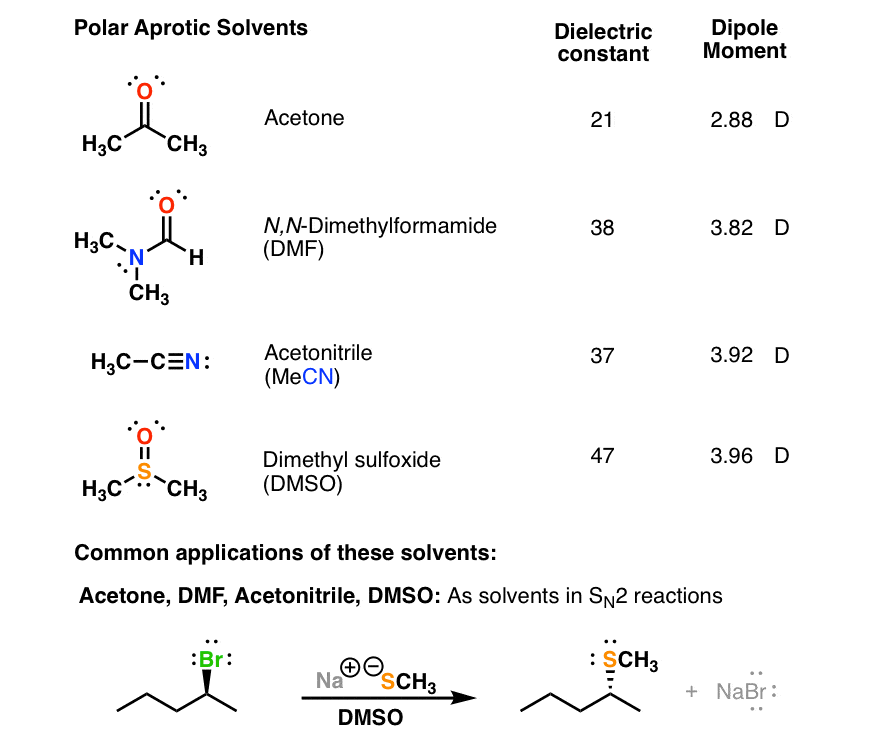
Polar Protic Polar Aprotic Nonpolar All About Solvents
Polar Vs Nonpolar Solvents Identifications And Examples Psiberg
0 Response to "Acetone Polar or Nonpolar Solvent"
Post a Comment